Abstract
Abstract
Background:
Venous thromboembolism (VTE) is an important immune-related adverse event (irAE) associated with immune checkpoint inhibitors (ICI) cancer therapy. To better understand the pathogenesis of ICI-induced VTE during ICI treatment, we utilized a murine model of cancer associated thrombosis (CAT) to examine the impact of ICI treatment on the development of flow restriction-induced thrombosis.
Methods
A syngeneic colon carcinoma murine model (CT26) on BALB/c background was utilized to evaluate venous thrombosis following inferior vena cava (IVC) ligation. Non-tumor-bearing and tumor-bearing animals were treated with therapeutic doses of ICI: anti-programmed cell death receptor 1 antibody (anti-PD1) and cytotoxic T‐lymphocyte-associated protein 4 (anti‐CTLA4) or isotype control antibodies. Mice underwent surgical treatment for IVC ligation followed by surgical retrieval of thrombus. Western blot analysis was performed on plasma cleaved high molecular weight kininogen (cHK), and citrullinated histone H3 (CitH3).
Results
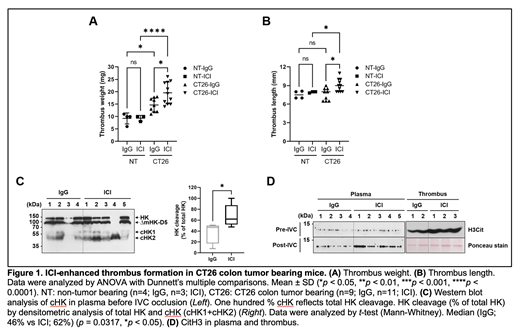
Tumor-bearing mice undergoing IVC ligation after anti-PD1 and anti-CTLA4 infusion developed larger thrombi compared to mice treated with isotype control IgG. Thrombus weights in CT26 tumor-bearing mice treated with ICI (19.56±4.41 mg) were significantly increased compared to those in mice treated with isotype control IgG (14.67±2.76 mg) (P=0.043). The weight of thrombi in non-tumor-bearing mice was not affected by ICI treatment (9.25±2.22 mg with control IgG vs 9.33±1.15 mg with ICI). In addition, there was a significant increase in thrombus length in mice treated with ICI (9±1.02 mm vs 7.61±0.99 mm with IgG, P=0.039). Cleaved high molecular weight kininogen (cHK), an indicator of contact activation was increased in plasma pre-IVC occlusion from ICI-treated mice compared to IgG-treated mice (68% vs 38% in HK cleavage). Citrullinated histone H3 (CitH3), a NETosis marker, was elevated in plasma (and in thrombus) post-IVC occlusion from ICI-treated mice compared to that of IgG-treated mice.
Conclusions
ICI treatment of tumor-bearing mice undergoing flow restriction-induced thrombosis resulted in enhanced clot formation. Larger thrombi in ICI treated mice was accompanied with enhanced contact activation and NETosis marker CitH3.
Khorana: Halozyme: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Anthos: Consultancy, Honoraria. McCrae: Dova, Novartis, Rigel, and Sanofi Genzyme: Consultancy; Sanofi, Novartis, Alexion, and Johnson & Johnson: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal